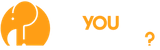A large number of blood pressure medications have been recalled over the last few months. Check below to see if this recall affects you, provided by the FDA.
| Lot # | Exp. Date | Product Description / Strength | Bottle Size | NDC |
|---|---|---|---|---|
| 23X017 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 90 Count | 0093-7690-98 |
| 23X018 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 30 Count | 0093-7690-56 |
| 23X018 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 90 Count | 0093-7690-98 |
| 23X019 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 30 Count | 0093-7690-56 |
| 23X019 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 90 Count | 0093-7690-98 |
| 23X020 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 30 Count | 0093-7690-56 |
| 23X022 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 30 Count | 0093-7690-56 |
| 23X023 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 30 Count | 0093-7690-56 |
| 23X023 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 90 Count | 0093-7690-98 |
| 23X024 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/160 mg | 90 Count | 0093-7690-98 |
| 24X012 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/160 mg | 30 Count | 0093-7691-56 |
| 24X012 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/160 mg | 90 Count | 0093-7691-98 |
| 24X013 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/160 mg | 30 Count | 0093-7691-56 |
| 25X028 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 90 Count | 0093-7692-98 |
| 25X029 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 25X029 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 90 Count | 0093-7692-98 |
| 25X030 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 25X031 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 25X032 | 11/2018 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 25X035 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 25X037 | 4/2019 | Amlodipine and Valsartan Tablets 5 mg/320 mg | 30 Count | 0093-7692-56 |
| 26X036 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 90 Count | 0093-7693-98 |
| 26X038 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 90 Count | 0093-7693-98 |
| 26X039 | 11/2018 | Amlodipine and Valsartan Tablets | 30 Count | 0093-7693-56 |
| Lot # | Exp. Date | Product Description / Strength | Bottle Size | NDC |
|---|---|---|---|---|
| 10 mg/320 mg | ||||
| 26X039 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 90 Count | 0093-7693-98 |
| 26X040 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X041 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X042 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X043 | 11/2018 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X044 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 90 Count | 0093-7693-98 |
| 26X045 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 90 Count | 0093-7693-98 |
| 26X046 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X047 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X048 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X049 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X050 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| 26X051 | 4/2019 | Amlodipine and Valsartan Tablets 10 mg/320 mg | 30 Count | 0093-7693-56 |
| Lot # | Exp. Date | Product Description/ Strength | Bottle Size | NDC |
|---|---|---|---|---|
| 18X010 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 5 mg/160 mg/12.5 mg | 30 count | 0093-7807-56 |
| 18X010 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 5 mg/160 mg/12.5 mg | 90 count | 0093-7807-98 |
| 18X011 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 5 mg/160 mg/12.5 mg | 30 count | 0093-7807-56 |
| 20X006 | 11/2018 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/160 mg/12.5 mg | 30 count | 0093-7810-56 |
| 20X006 | 11/2018 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/160 mg/12.5 mg | 90 count | 0093-7810-98 |
| 21X006 | 11/2018 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/160 mg/25 mg | 30 count | 0093-7038-56 |
| 21X006 | 11/2018 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/160 mg/25 mg | 90 count | 0093-7038-98 |
| 21X007 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/160 mg/25 mg | 30 count | 0093-7038-56 |
| 22X045 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/320 mg/25 mg | 30 count | 0093-7809-56 |
| 22X045 | 2/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/320 mg/25 mg | 90 count | 0093-7809-98 |
| 22X046 | 02/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/320 mg/25 mg | 30 count | 0093-7809-56 |
| 22X047 | 02/2019 | Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 10 mg/320 mg/25 mg | 30 count | 0093-7809-56 |
Please SHARE this article to spread awareness about this widespread recall!
Check out the full news coverage on the first slew of recalls from July of 2018 below.
Pages: Page 1 Page 2